UKCA Marking
Date: 16 March 2022
UKCA marking came into force in Great Britain in January 2021 when the UK left the European Union. UKCA Certification may be required for certain classifications of medical devices and is available from the UK appointed Approved Bodies, such as BSI (0086). There is a transition period to 30 June 2023 to allow existing CE certifications to be replaced by the new UKCA mark. However, the consultation for the new UK legislation has ended; therefore, the new UK legislation should be published later this year.
From 1 January 2021, medical devices/IVDs must be registered with the MHRA before being placed on the UK market irrespective of whether UKCA marked or CE marked. The grace periods for the registration of devices based on their risk class have now ended. Therefore, it is mandatory to register all devices with the MHRA before placing them on the market.
For Northern Ireland, the EU MDR and IVDR apply from 26 May 2021 and 26 May 2022, respectively. Even after 1 July 2023, a CE mark will continue to be required for devices placed on the Northern Ireland market and manufacturers will need to meet EU regulations.
We want to clarify a few other aspects of UKCA in this communication. Firstly, please refer to our detailed FAQ document online, which covers many questions you may have around UKCA.
- Labelling and IFU requirements for UKCA marking
It is critical to ensure that your labelling and IFUs have been updated to reference your UKCA mark if this is relevant. The primary considerations for this area include:
- Labelling must reference the UKCA marking, including the approved body number, for example, BSI (0086), if an approved body has been involved in the conformity assessment process
- Devices can have both the CE and UKCA markings present on the labelling prior to 1 July 2023, and dual marking will continue to be accepted on the Great Britain market after 1 July 2023.
- UK Responsible Person
A UK Responsible Person (UKRP) must be appointed if the manufacturer is based outside the UK. This is a mandatory requirement for all UKCA or CE marked products for non-UK manufacturers. Please refer to the MHRA requirements for the UKRP.
- Declaration of Conformity (DoC) required for UKCA marking
In cases where the UKCA marking has been affixed (including when devices have been dual marked), your DoC needs to be updated for UKCA marking. The DoC should reference UK legislative requirements, including references; the correct legislation is Medical Devices Regulations 2002 (SI618) as subsequently amended by the EU Exit Regulations of 2019 (SI 791) and 2020 (SI 1478).
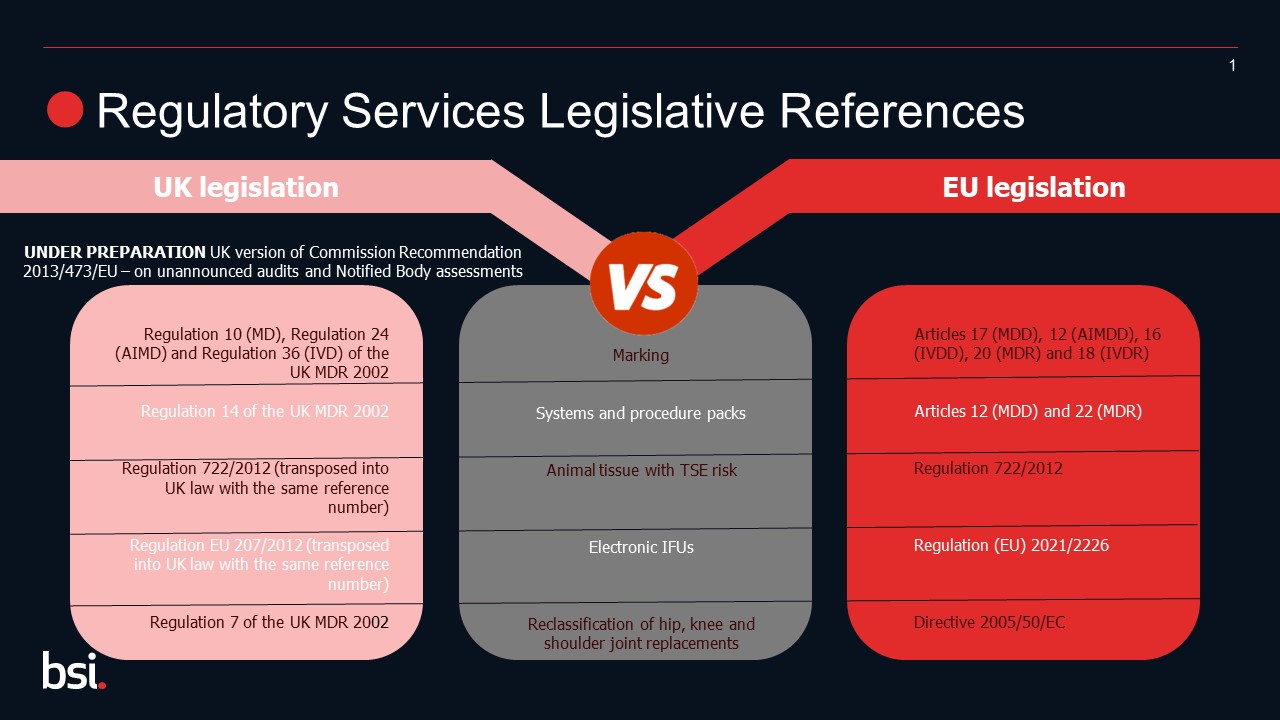
- UK horizontal legislation and designated standards
Horizontal legislation references have changed as part of UKCA; please refer to the diagram to assess your requirements.
Moreover, when the UK left the EU, the link with the harmonized standards published in the EU Official Journal was broken. As a result, the UK has published its own list of standards that support UK regulation. These have been termed "designated standards".
Manufacturers using standards to support the conformity of their devices should be aware of the publication of the UK lists of designated standards and monitor for further information on maintenance of these lists.
What do I need to do now?
CE marking will cease to be accepted for the UK market post-June 2023, and UKCA marking will become mandatory from July 2023. Therefore, ensure your submissions contain updated information so as not to cause undue delay in reviews.
BSI would like to ask you about your current plans to allow us to assign the required technical capacity. Please could you take 10 minutes to complete our survey to support our resource planning as a UKCA Approved Body?
Tell us about your UKCA Plans by completing this short questionnaire.
Please complete this by Friday 1 April 2022.
Who can I contact for further information?
We will be running a webinar on 27 April to share the latest updates; please join us live.
If you have questions or concerns, please refer to the BSI website in the first instance, where we maintain up to date information on UKCA certification. Further information can be provided via your BSI Scheme Manager if needed.
Yours Sincerely,
Vishal Thakker
Head of UK Approved Body,
Regulatory Services (Medical Devices)



